n3- protons neutrons electrons|Number of Protons, Neutrons, and Electrons in an Atom : Tuguegarao Elements are organised into blocks by the orbital type in which the outer electrons are found. These blocks are named for the characteristic spectra they produce: sharp (s), .
AZNude has a global mission to organize celebrity nudity from television and make it universally free, accessible, and usable. We have a free collection of nude celebs and movie sex scenes; which include naked celebs, lesbian, boobs, underwear and butt pics, hot scenes from movies and series, nude and real sex celeb videos.
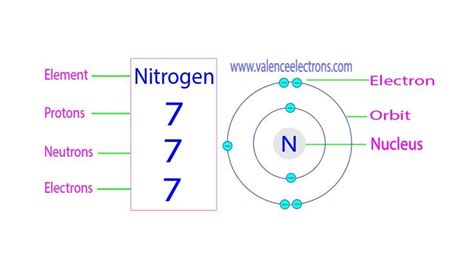
n3- protons neutrons electrons,Protons and neutrons are found in the nucleus, the dense region at the center of an atom. Electrons are found outside the nucleus. Protons are positively charged and have a mass of about 1 u. Neutrons are neutral (have no charge) and also have a mass of .Solution. Verified by Toppr. Correct option is A. 7 protons and 10 electrons. Was this answer helpful? 3. Similar Questions. Q 1. The nitrogen atom has 7 protons and 7 . Describe the locations, charges, and masses of the three main subatomic particles. Determine the number of protons and electrons in an atom. Write and .

Neutral atoms have the same number of electrons and protons. Atoms of an element that contain different numbers of neutrons are called isotopes. Each isotope of a given element has the same atomic .
Know the basics of the experiments involving the discoveries of the three subatomic particles. Memorize relative charge values and amu masses of the three subatomic .n3- protons neutrons electronsElements are organised into blocks by the orbital type in which the outer electrons are found. These blocks are named for the characteristic spectra they produce: sharp (s), .

Follow these simple steps to find the number of protons, neutrons, and electrons for an atom of any element. Key Takeaways: Number of Protons, Neutrons, and Electrons. Atoms are made of .
Determine the number of electrons. Protons are particles in the nucleus of an atom that have a positive charge equal to +1. Electrons are particles that have a negative charge equal to -1. .Table of Relative Charge & Mass. If have a particle has 0 relative charge, this means it is neutral. Both the proton and neutron relative mass is 1, this means that they have the . A atom M have 82 protons, 126 neutrons and 82 electrons, another species N contain 82 protons, 126 neutrons and 80 electrons, then N is a : Medium. View solution > Calculate the total number of electrons present in 1. 4 .2. Indique le nombre de protons, électrons, neutrons contenus dans les éléments suivants : 15 N 33 S 63 Cu 84 Sr 130 Ba 186 W 202 Hg 7 16 29 38 56 74 80 Protons 7 16 29 38 56 74 80 Électrons 7 16 29 38 56 74 80 Neutrons 8 17 34 46 74 112 122 3. Donne le symbole approprié pour chacun des isotopes suivants : 1. Z = 11 et A = 23 Na 2. Z = 28 .
Calculate numbers of protons, neutrons, and electrons by using mathematical expressions (1-3): p = 11 (1) n = 23 - 11 = 12 (2) e = 11 - 0 = 11 (3) Alternatively, you can also calculate the atomic number, atomic mass, and charge. Choose your element. Let's assume that it is the sulfide anion. Find the numbers of protons, .Watch this video to learn how protons, neutrons, and electrons are arranged in atoms, and how the number and distribution of these subatomic particles determine the identity and properties of .n3- protons neutrons electrons Number of Protons, Neutrons, and Electrons in an Atom Make sure that you round the atomic mass to the nearest whole number. For example, the atomic mass of boron is 10.811, but you can just round the atomic mass up to 11. 6. Subtract the atomic number from the atomic mass. To find the number of neutrons, you will need to subtract the atomic number from the atomic mass.While protons and neutrons are located inside the nucleus at the center of the atom, electrons are located outside the nucleus in what is often called the electron cloud. Figure 4.4.1 4.4. 1: Electrons are much smaller than protons or neutrons. If an electron was the mass of a penny, a proton or a neutron would have the mass of a large bowling .Number of Protons, Neutrons, and Electrons in an Atom L’atome est donc essentiellement composé de vide. Ce qu’il faut retenir : la masse d’un proton et d’un neutron est quasiment la même. Mais un électron est environ 1000 fois plus léger : la masse des électrons est donc négligeable par rapport à celle des protons. Ainsi la masse de l’atome est concentrée dans le noyau.
Table 4.4.1 4.4. 1 gives the properties and locations of electrons, protons, and neutrons. The third column shows the masses of the three subatomic particles in "atomic mass units." An atomic mass unit (amu amu) is defined as one-twelfth of the mass of a carbon-12 atom. Atomic mass units ( amu amu) are useful, because, as you can .
n3- protons neutrons electrons|Number of Protons, Neutrons, and Electrons in an Atom
PH0 · The nitrogen atom has 7 protons and 7 electrons, the nitride
PH1 · The nitrogen atom has 7 protons and 7 electrons, the nitride
PH2 · Protons, neutrons, and electrons in atoms (video)
PH3 · Number of Protons, Neutrons, and Electrons in an Atom
PH4 · Number of Protons, Neutrons, and Electrons in an Atom
PH5 · Nitrogen
PH6 · How to find Protons & Electrons for the Nitride ion (N 3
PH7 · How to Find the Number of Protons, Neutrons, and Electrons
PH8 · How to Find the Number of Protons, Neutrons, and
PH9 · 5.1.3 Protons, Neutrons & Electrons
PH10 · 3.3: Subatomic Particles
PH11 · 2.6: Protons, Neutrons, and Electrons in Atoms
PH12 · 1.8: Subatomic Particles